Hot-melt applications for the food and beverage industry – How to safely stabilize vitamins and probiotics
Sensitive substances such as probiotics, vitamins or sustained release dosage forms must be furnished with a functionally protective coating to ensure their safe transport through the intestinal tract. Furthermore, the surface treatment guarantees their release at a well-defined point in time. Hot-melt fluidized bed granulation provides a variety of possibilities for product design.
- Author: Dr. Michael Jacob, Head of Process Technology Food, Feed & Fine Chemicals, Glatt Ingenieurtechnik GmbH
- originally published in the trade magazine ‘foodeurope’, issue 01/2017, Hoskins & Fall Publishing
The demand for health-promoting foods and so-called functional foods has been increasing for years. In drug production, active ingredients are embedded in pharmaceutical formulations using hot-melt extrusion. A similar principle exists for nutraceuticals and functional foods: hot-melt fluidized bed granulation. Glatt Ingenieurtechnik, a globally operating engineering specialist from Weimar, Germany, develops appropriate processes and plants, and also designs production sites.
Active ingredients pose many challenges for product developers: in conventional freeze-drying or vacuum drying, for example, certain microorganisms, such as Bifidus bifidum, perish. In addition, their poor flowability and the uneven grain size distribution are adversely affected by further processing. The pharmaceutical industry struggles with similar challenges: more than a third of currently available active ingredients are difficult to dissolve and are therefore poorly bioavailable — and the trend is rising. The active ingredients of the future will be largely insoluble in water and difficult to absorb. Processes such as melt extrusion or fluidized-bed micro encapsulation can be used to encapsulate valuable solid or liquid ingredients in a homogenous granulate and provide controlled release. Compared with extrusion, hot-melt granulation by fluidized bed processing allows a wider bandwidth in particle design, including the shear-free integration of additives and surface functionalization.
The fluidized bed principle
One of the essential advantages of fluidized bed technology is its intensive process control, which allows several steps — such as drying or solidifiction and product design — to be done in the same apparatus. This is very economical and makes it possible to thermally dry/solidify, refine and functionalize raw materials in a single process step (agglomeration, coating and encapsulation, for example). It is the unique flow and thermodynamic properties of fluidized and spouted bed technologies that have established themselves among the pioneering processes in the formulation and optimization of powder properties and as particle-forming methods for solids-containing liquids – e.g. melts, dispersions, solutions, and suspensions.
Coating using fluidized bed technology
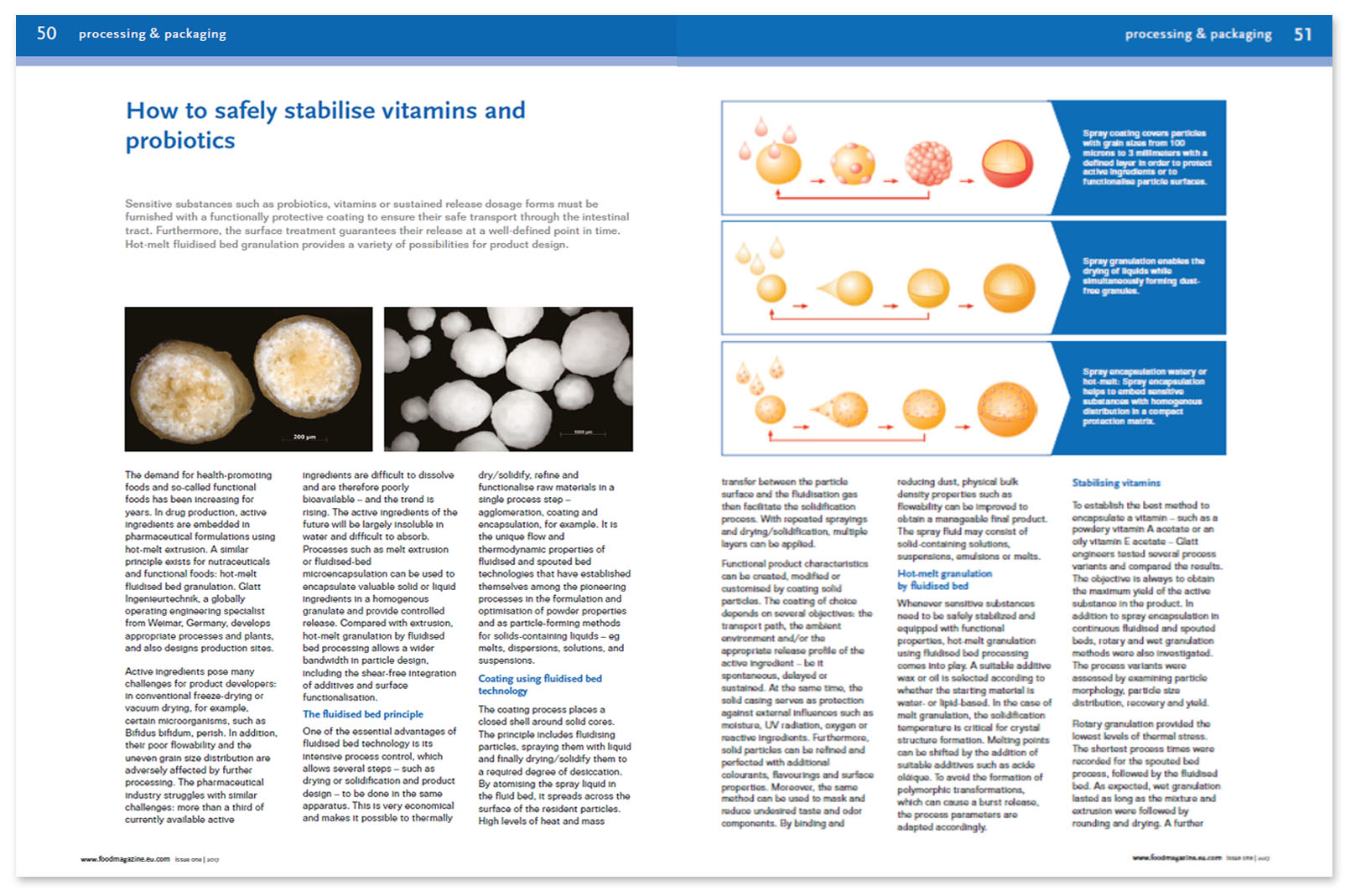
The coating process places a closed shell around solid cores. The principle includes fluidizing particles, spraying them with liquid and finally drying/solidify them to a required degree of desiccation. By atomizing the spray liquid in the fluid bed, it spreads across the surface of the resident particles. High levels of heat and mass transfer between the particle surface and the fluidization gas then facilitate the solidification process. With repeated sprayings and drying/solidification, multiple layers can be applied.
Functional product characteristics can be created, modified or customized by coating solid particles. The coating of choice depends on several objectives: the transport path, the ambient environment and/or the appropriate release profile of the active ingredient — be it spontaneous, delayed or sustained. At the same time, the solid casing serves as protection against external influences such as moisture, UV radiation, oxygen or reactive ingredients. Furthermore, solid particles can be refined and perfected with additional colorants, flavorings and surface properties. Moreover, the same method can be used to mask and reduce undesired taste and odor components. By binding and reducing dust, physical bulk density properties such as flowability can be improved to obtain a manageable final product. The spray fluid may consist of solid-containing solutions, suspensions, emulsions or melts.
Hot-melt granulation by fluidized bed
Whenever sensitive substances need to be safely stabilized and equipped with functional properties, hot-melt granulation using fluidized bed processing comes into play. A suitable additive wax or oil is selected according to whether the starting material is water- or lipid-based. In the case of melt granulation, the solidification temperature is critical for crystal structure formation. Melting points can be shifted by the addition of suitable additives such as acide oléique. To avoid the formation of polymorphic transformations, which can cause a burst release, the process parameters are adapted accordingly.
Stabilizing vitamins
To establish the best method to encapsulate a vitamin – such as a powdery vitamin A acetate or an oily vitamin E acetate — Glatt engineers tested several process variants and compared the results. The objective is always to obtain the maximum yield of the active substance in the product. In addition to spray encapsulation in continuous fluidized beds and spouted beds, rotary and wet granulation methods were also investigated. The process variants were assessed by examining particle morphology, particle size distribution, recovery and yield.
Rotary granulation provided the lowest levels of thermal stress. The shortest process times were recorded for the spouted bed process, followed by the fluidized bed. As expected, wet granulation lasted as long as the mixture and extrusion were followed by rounding and drying. A further observation was that the particles looked very different, depending on whether they were encapsulated within a water-based or a lipid-based formulation. If the encapsulation was done by fluidized or spouted bed processing under a nitrogen atmosphere in a closed cycle operation, better recovery rates were recorded. By adjusting the product temperature, spray rate and spray pressure in the fluid bed, it is possible to influence the shape, structure and size of the resulting particles. However, parameters and results of batch-based processing cannot be transferred easily to continuous processing. Continuous processes, by contrast, are energetically more effective and easier to automate.
Stabilizing probiotics
Glatt Ingenieurtechnik has developed and patented a special process for the encapsulation and immobilization of microorganisms. The benefit of the process lies not only in the advantageous heat and mass transfer conditions, which is why the particle surfaces uniformly moisten and become regular granules. The company’s ProCell series apparatus allows the microorganisms to be dried at a much higher temperature than conventional drying. As a rule, the liquid formulation containing the microbial cultures consists of an aqueous solution. In trials, carrier materials (maltodextrin and whey powder) were applied to provide protection and improve the shelf-life. An additional symbiotic effect can be provided by adding prebiotic fibers into the capsules. Interestingly, it is possible to apply a functional coating in the same process. In addition, cleaning is simple and hygienic, so that the cross-contamination of biological substances during product changeovers can be eliminated.
Starting small
In conclusion, it is most economical to start small and do tests on a laboratory plant with lower quantities of raw materials. With the help of in-process analyses of the active substance or other important particle characteristics — such as size and bulk density — procedures can be quickly adapted to obtain the desired product properties. Glatt provides test facilities with various process operations, system configurations and laboratory equipment at its Technology Centre in Weimar, Germany. A team of experienced food and process engineering experts is available throughout the usually week-long test series. For reliable scale-up to production scale — especially useful for continuous processing— local pilot plants are also available.
Further information on this topic and related topics can also be found in the following publications:
Published article: ‘How to Tame Recalcitrant Ingredients with Technological Processes’ PDF, English
Published article: ‘The gentle processing of highly volatile oils by fluid bed and spouted bed technology’ PDF, English
Published article: ‘Spray (micro)encapsulation of sensitive substances in matrix form – An overview of essential oil and vitamin case studies’ PDF, English
Published article: ‘Optimizing end products with finely tuned process parameters’ PDF, English
Published article: ‘Customised enzymes for optimal animal feed mixes’ PDF, English






 Copyright: publish-industry Verlag GmbH
Copyright: publish-industry Verlag GmbH Copyright: Dr. Harnisch Verlagsgesellschaft mbH
Copyright: Dr. Harnisch Verlagsgesellschaft mbH